Why does solid sodium chloride act as a non-electrolyte while an aqueous NaCl solution acts as a strong electrolyte? - Quora

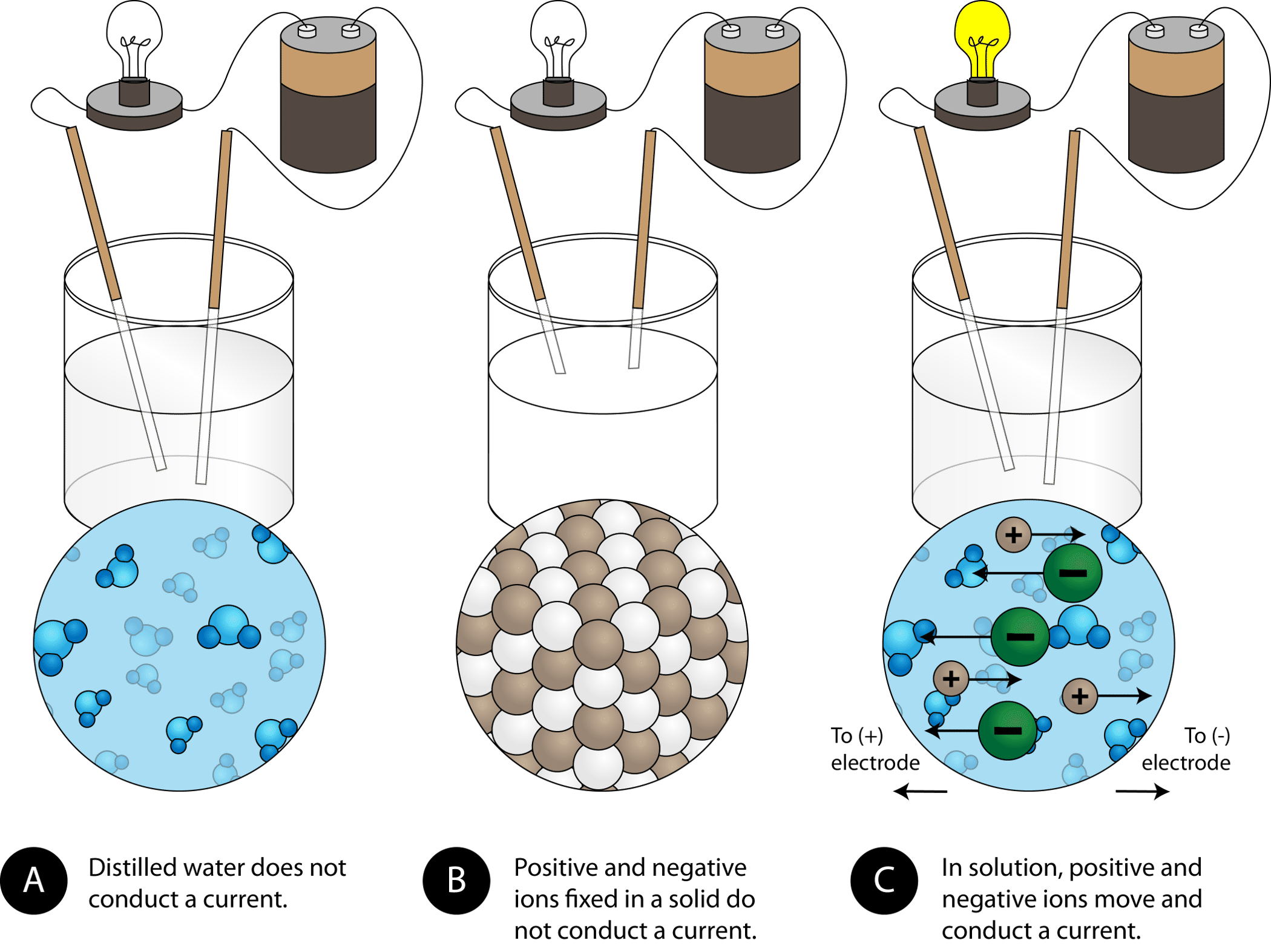
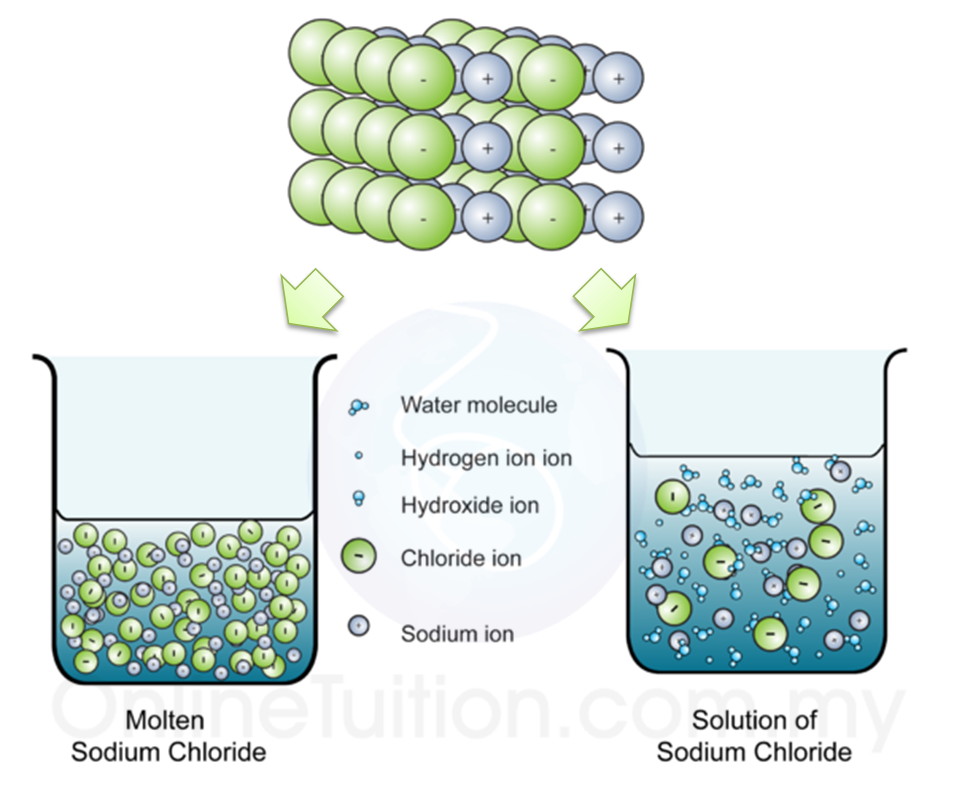
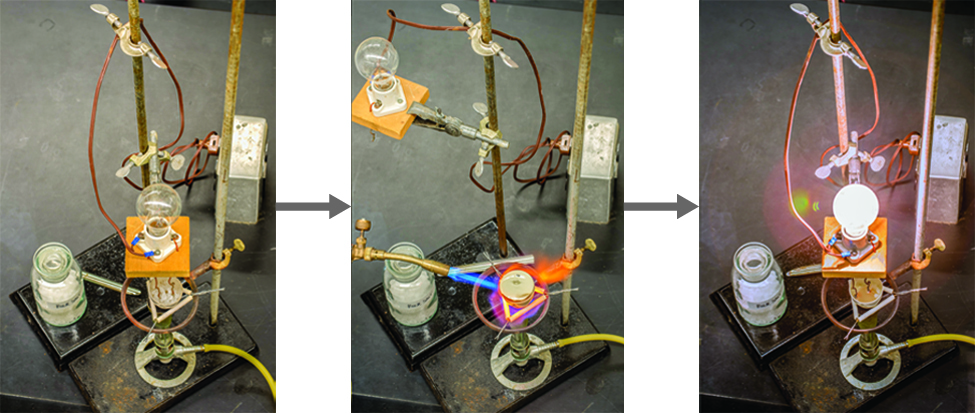
Explain the following : (a) Sodium chloride is an ionic compound which does not conduct electricity in solid state whereas it does conduct electricity in molten state as well as in aqueous

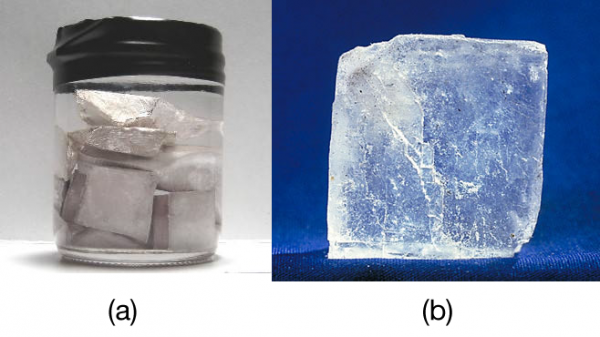
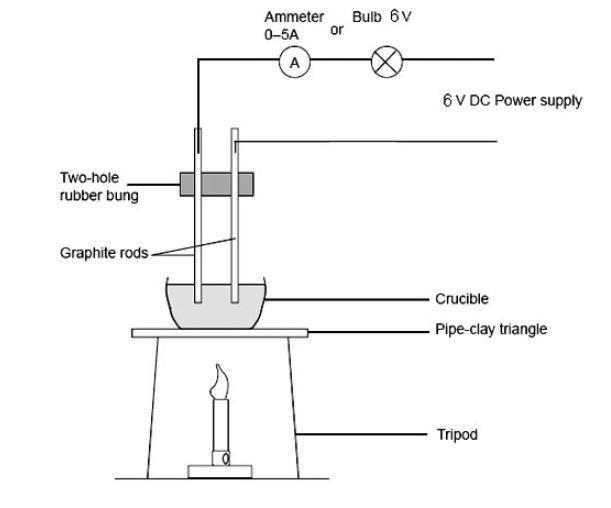
Chapter 4 Formation of Compounds. Properties of Salt White solid at room temperature Crystal shaped cubes Hard & brittle Solid salt does not conduct electricity. - ppt download

√ A solid salt crystal is in insulator. Explain how salts can be made to conduct electricity - YouTube

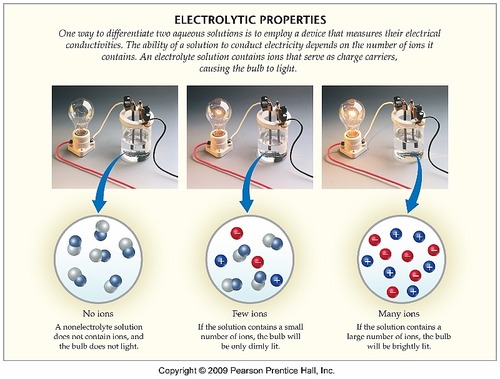
On Target? Do this on your Warm Up worksheet! Why does sugar dissolved in water not conduct electricity, while salt dissolved in water does conduct electricity? - ppt download