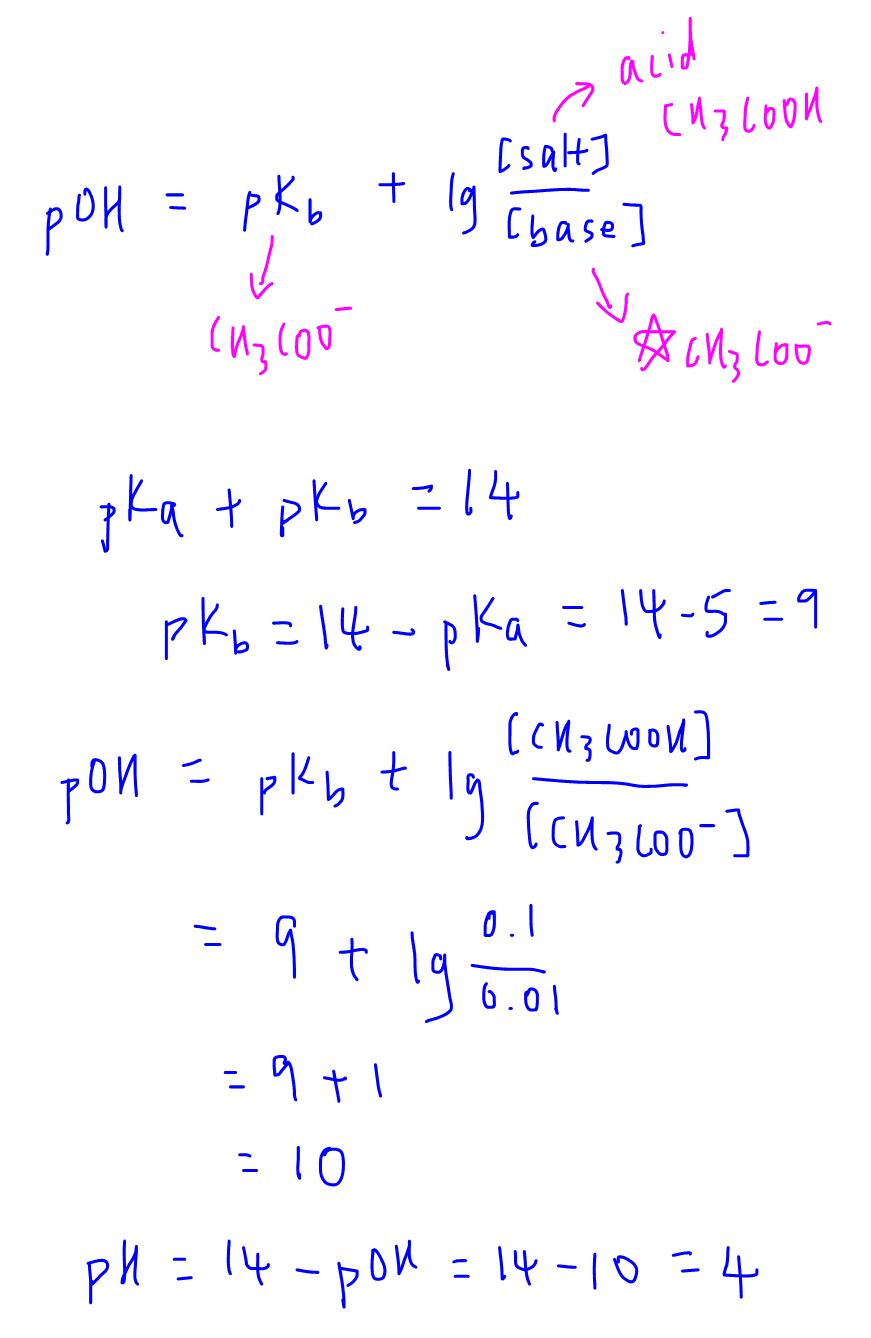We'll show you how to calculate the pH of a solution formed by mixing a strong acid with a strong base. Strong Acid– Strong Base Mixtures. - ppt download

Calculate the pH of the resultant mixtures:a) 10 mL of 0.2M Ca (OH)2 + 25 mL of 0.1M HCl b) 10 mL of 0.01M H2SO4 + 10 mL of 0.01M Ca (OH)2

Calculate the pH of the following mixture 50mL of 0.05M CH(3)COOH+50mL of 0.05M NH(4) OH Given : pK(a)=pK(b)=4.74

equilibrium - Calculation of the pH of a mixture of a strong acid and weak acid - Chemistry Stack Exchange

Calculate the pH of the resultant mixture: a. `10 mL` of `0.2M Ca(OH)_(2)+25 mL` of `0.1 M HCl` ... - YouTube

Calculate the ph of a solution formed by mixing equal volumes of two solutions A and B of a strong acids having ph=6" and "ph=4 respectively.