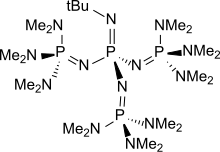
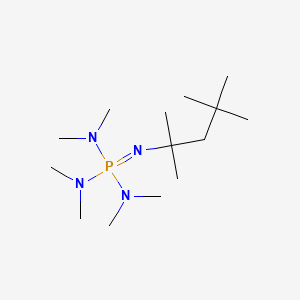
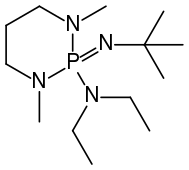
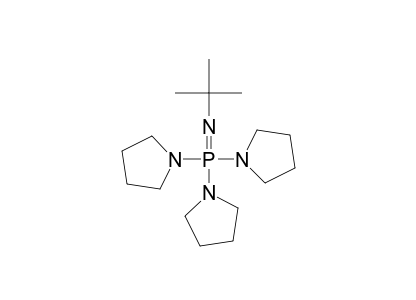
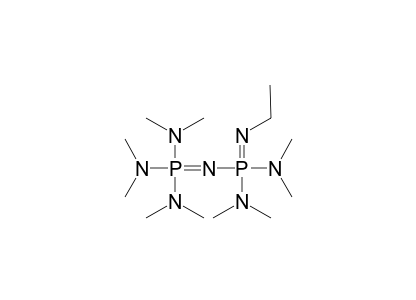
Deprotonation of benzylic ethers using a hindered phosphazene base. A synthesis of benzofurans from ortho-substituted benzaldehydes. | Semantic Scholar

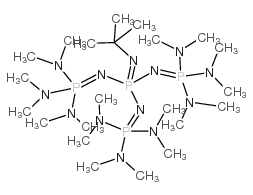
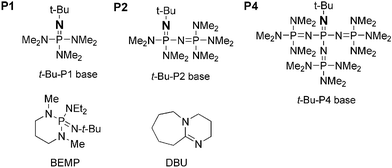
Scheme 1. Chemical structures of the phosphazene bases t-BuP1, t-BuP2... | Download Scientific Diagram

pK ip values of phosphazene bases 6a,b and several other representative... | Download Scientific Diagram
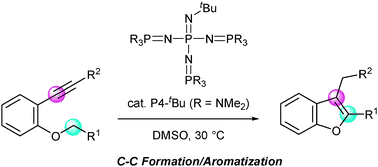
Phosphazene base-catalyzed intramolecular cyclization for efficient synthesis of benzofurans viacarbon–carbon bond formation - Chemical Communications (RSC Publishing)

Synthesis of end‐functionalized polyethers by phosphazene base‐catalyzed ring‐opening polymerization of 1,2‐butylene oxide and glycidyl ether - Misaka - 2012 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library

31 P { 1 H} NMR spectra of the pure (a) phosphazene base 2d and (b) PIL... | Download Scientific Diagram
Phosphazene base-promoted functionalization of aryltrimethylsilanes - Chemical Communications (RSC Publishing) DOI:10.1039/B611090H

Synthesis of Tris-Phosphazene Bases with Triazine as Core and Their Applications for Efficient Ring-Opening Alternating Copolymerization of Epoxide and Anhydride: Notable Effect of Basicity and Molecular Size | ACS Macro Letters