Molar enthalpy of vaporization of a liquid is 2.6 kJ. If boiling point of this liquid is `160^(@)c`, - YouTube

thermodynamics - Does adding salt to water decrease the latent heat of vaporization? - Physics Stack Exchange

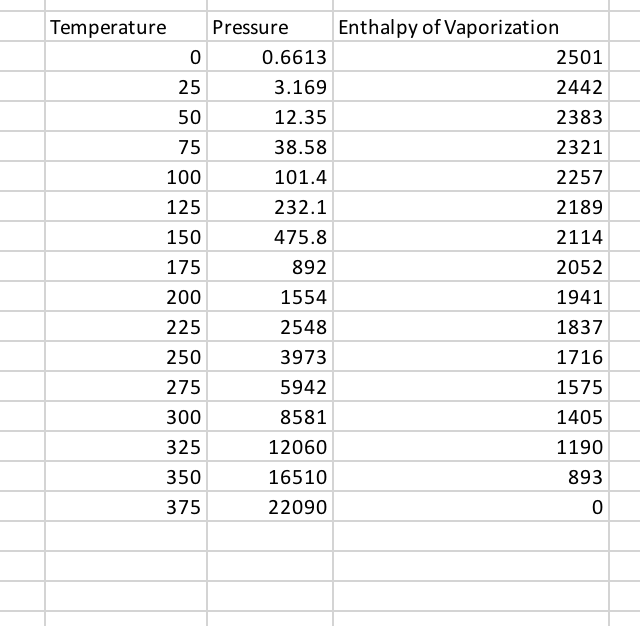
Enthalpy of vaporization of water: (—) Reference fundamental equation... | Download Scientific Diagram

SOLVED: The enthalpy of vaporization of water is 44 kJ/mol. How much heat would be released if 100 grams of water vapor condenses to liquid water? The answer is 244 kj but