QUICK GUIDE PHS HUMAN SUBJECTS & CLINICAL TRIALS INFORMATION NIH HUMAN SUBJECT STUDY RECORD ATTACHMENT
MIT Guide to PHS Human Subjects and Clinical Trial Information Form and Study Record for KC S2S and Workspace
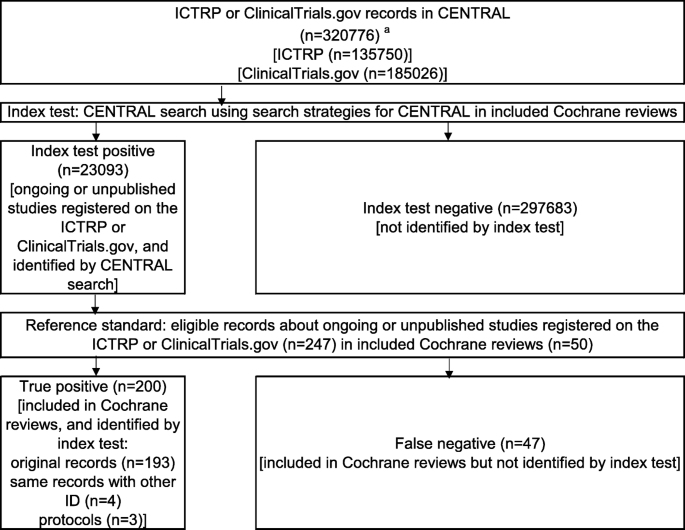
Using the Cochrane Central Register of Controlled Trials to identify clinical trial registration is insufficient: a cross-sectional study | BMC Medical Research Methodology | Full Text

Approach for reporting master protocol study designs on ClinicalTrials.gov: qualitative analysis | The BMJ

CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure | Zenosis – Learning for Life

Efficacy and safety of hydroxychloroquine as pre-and post-exposure prophylaxis and treatment of COVID-19: A systematic review and meta-analysis of blinded, placebo-controlled, randomized clinical trials. - The Lancet Regional Health – Americas
MIT Guide to PHS Human Subjects and Clinical Trial Information Form and Study Record for KC S2S and Workspace
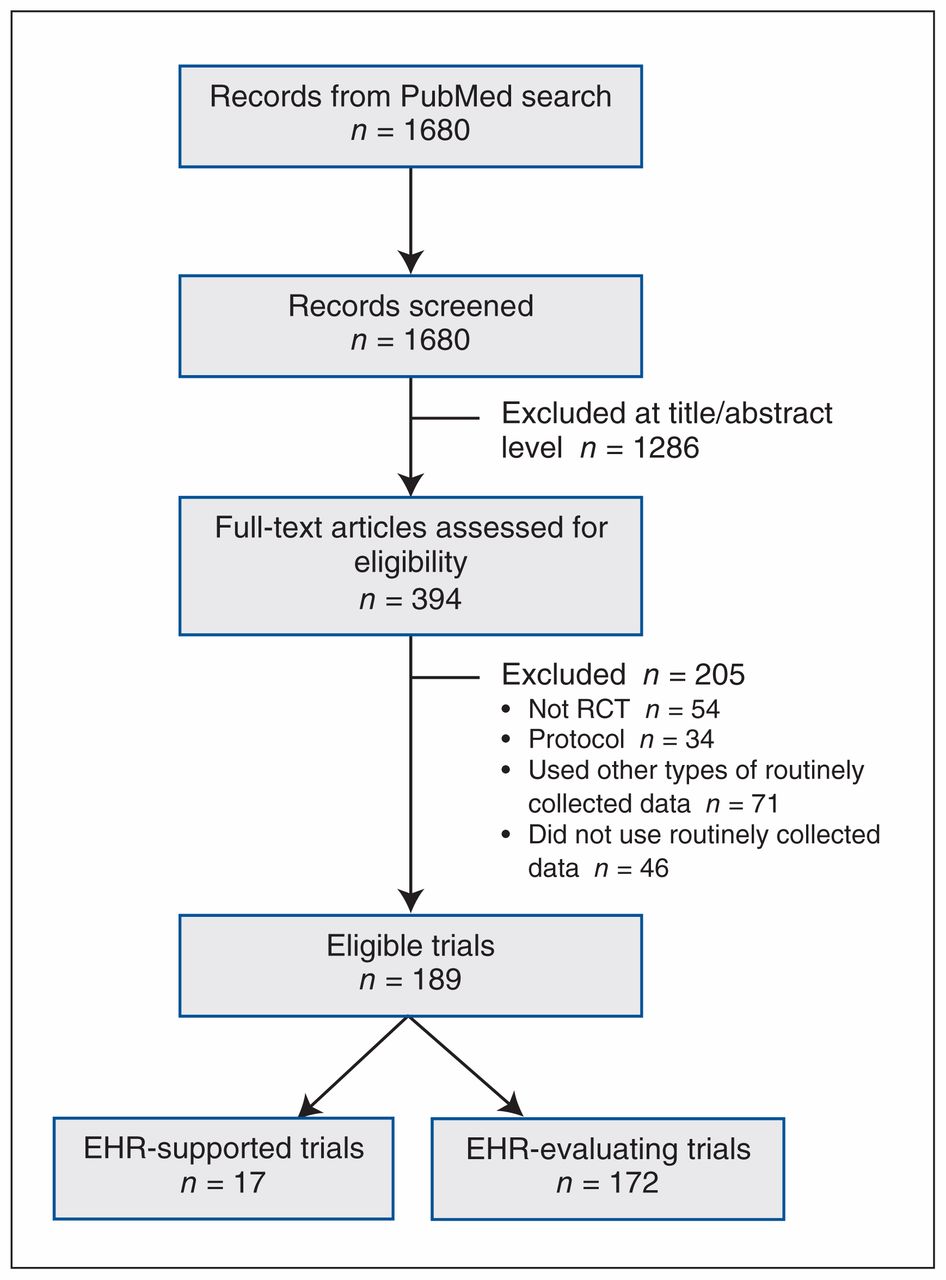
Current use and costs of electronic health records for clinical trial research: a descriptive study | CMAJ Open

Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action - Clinical Therapeutics