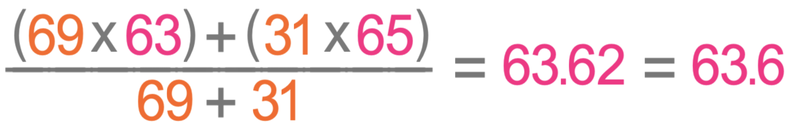An element has two isotopes with atomic masses (A - 1) and (A + 3) respectively. The average atomic mass is A . Calculate the mole percentage of the heavier isotope.

Defining how to calculate relative atomic mass of element relative isotopic mass definition gcse chemistry Calculations igcse O Level revision notes

Calculate mass of `Cu` in `3.67 xx 10^(3)g CuFeS_(2)` ? (Atomic mass `Cu = 63.5 Fe = 56,S = 32)` . - YouTube

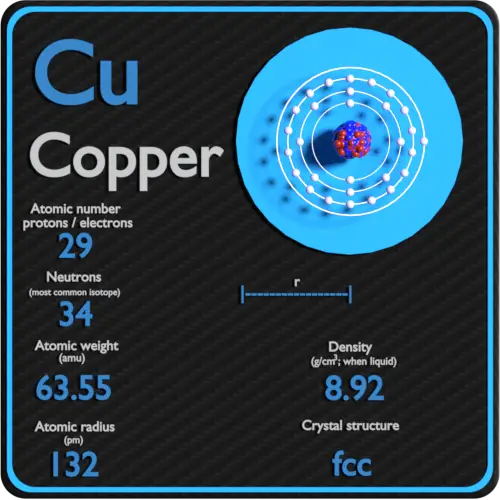
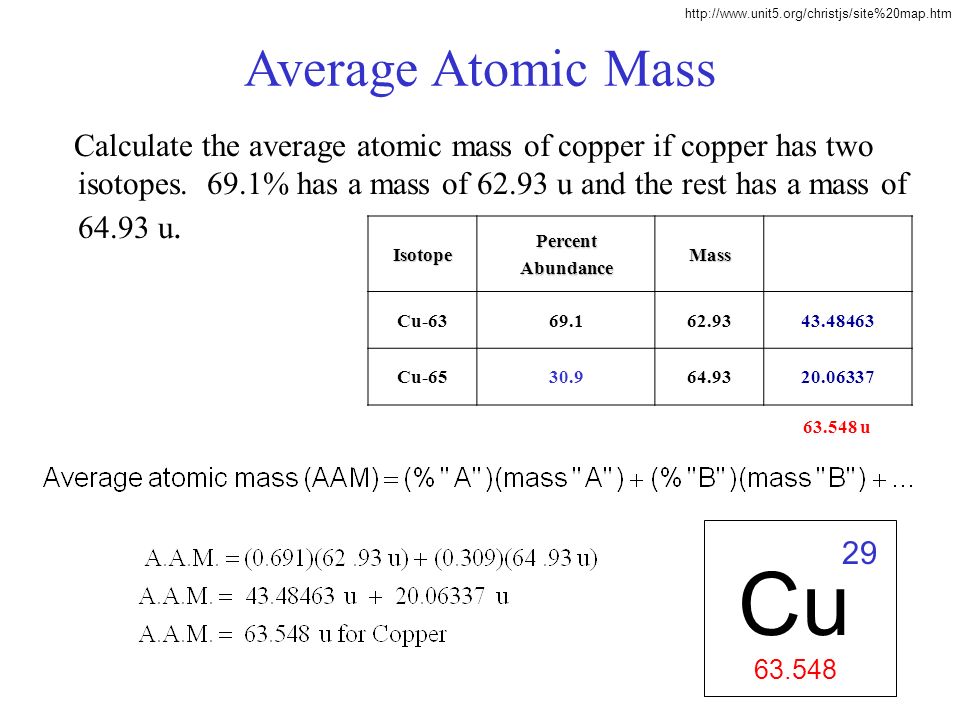
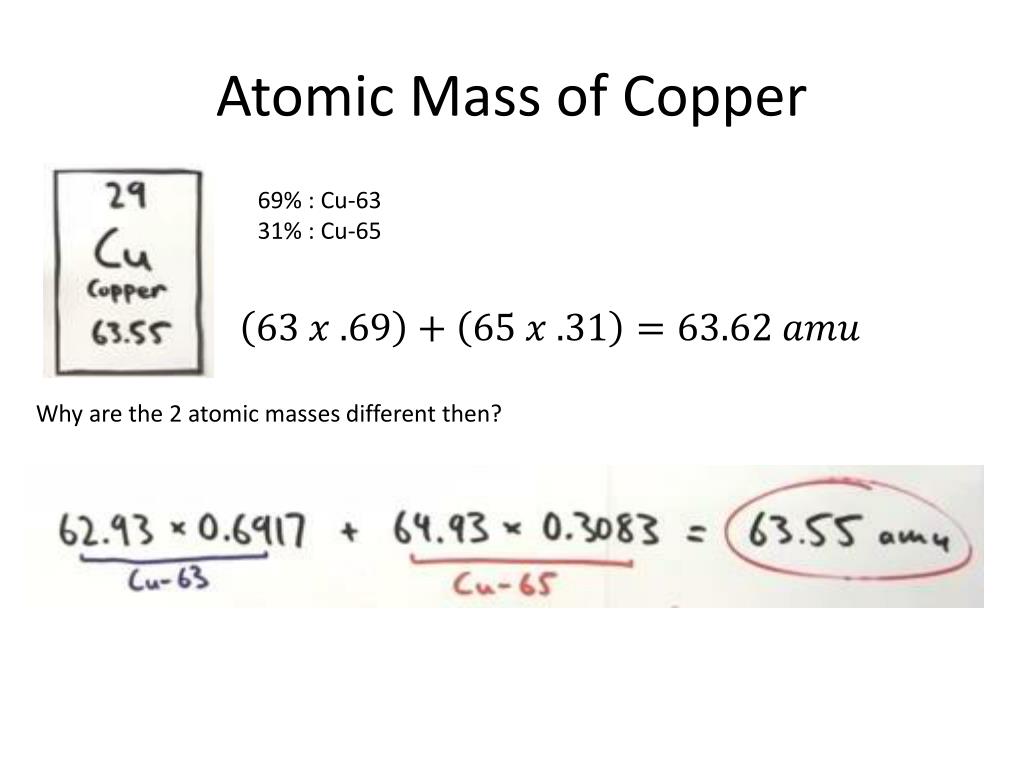
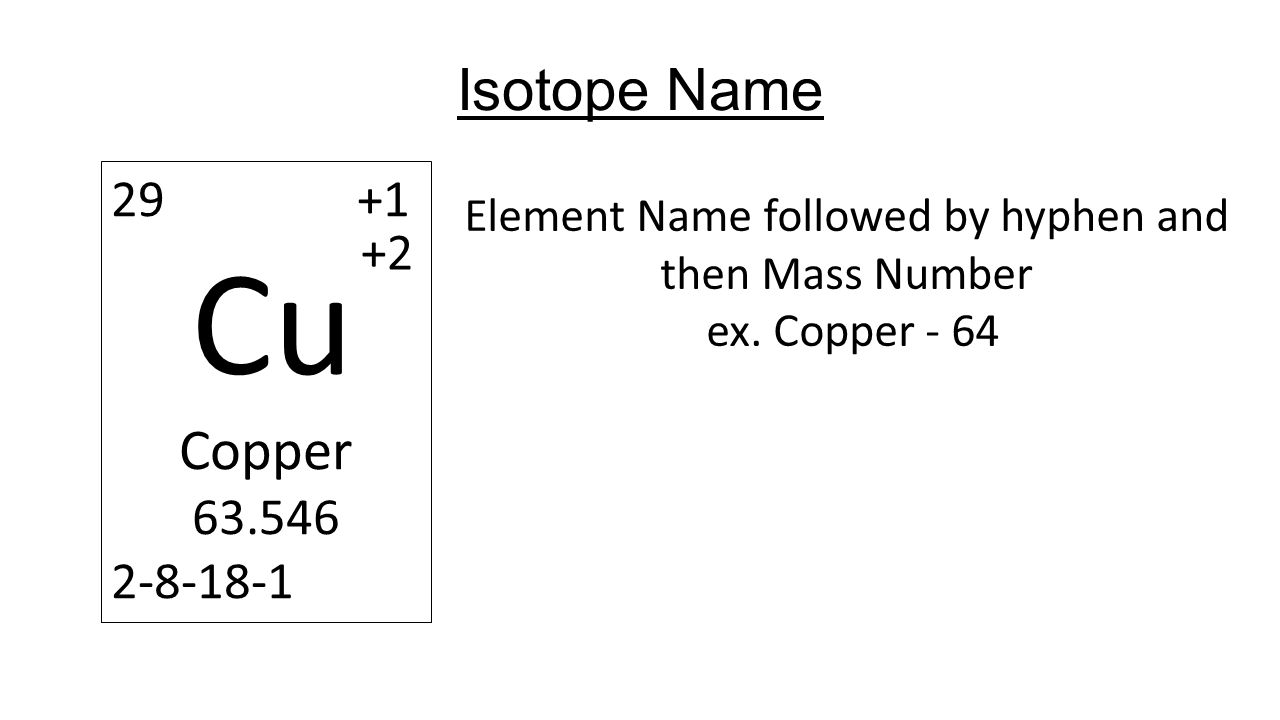
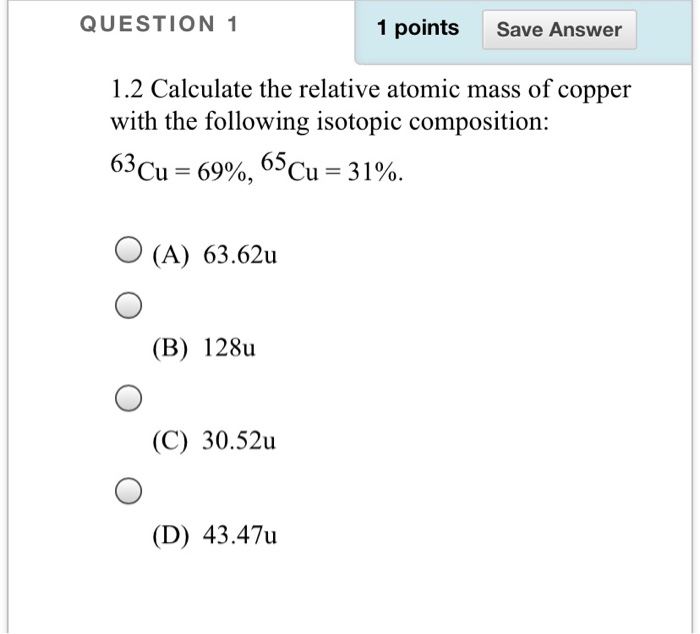
Cu has only two naturally occurring isotopes, 63Cu and 65Cu . If the atomic mass of Cu is 63.546 , then the natural abundance of the ^63Cu isotope will be approximately :
Cu Copper Chemical Element Periodic Table. Single vector illustration, element icon with molar mass, atomic number and electron conf Stock Vector Image & Art - Alamy